
Led from NUI Galway, BioInnovate Ireland is a national fellowship programme designed to facilitate innovation and entrepreneurship in the medical device sector in Ireland. Producing 28 medical device companies since its inception ten years ago, the fellowship programme continues to entice fellows from diverse backgrounds across clinical medicine, biomedical engineering, business development, industrial design and project management.
Here we meet some of the 2021 success stories.
Luminate Medical
Luminate Medical is a medical device NUI Galway spinout based in Galway, which has developed a novel technology to prevent the side effects of systemically delivered drugs. The company’s first product, Lily, is a headset device that prevents chemotherapy-induced hair loss in cancer patients.

Luminate Medical started back in 2019 when Aaron Hannon, Dr Bárbara Oliveira and Prof Martin O’Halloran, based in the Translational Medical Device Lab in the Lambe Institute, NUI Galway, understood how big of an unmet clinical need chemotherapy-induced hair loss is. In the US and Europe alone, 3 million cancer patients receive chemotherapy that causes hair loss every year, with a staggering 84% of patients already choosing to buy wigs, out of their own pocket. From a very early stage, the team conducted patient focus groups and hundreds of interviews and very quickly understood that hair loss is a need that deeply matters, first and foremost, to patients. Patients feel deprived of their sense of identity and privacy, and are at elevated risks of depression, anxiety and relationship breakdown.
The team was also quick to realise that current solutions for this huge clinical problem fail to meet patient needs. Existing devices are based on cryogenic therapy; however, pain, reliability and extended hospital stays are some of the concerns with the available cryogenic devices. Realising the limitations of current market offerings, the Luminate Medical team has developed and patented a novel therapeutic approach to prevent excess drug delivery to specific target tissues in the body, such as the hair. By preventing the delivery of chemotherapy to the hair in a comfortable, reliable and safe mechanism, hair loss is reduced, ultimately contributing to improving the patient experience through cancer treatment. Thus far, the team has validated their new therapeutic approach in animal and healthy volunteer studies, with very successful outcomes.

The team is committed to integrating the patients’ voices in the development process, so patient needs can truly be answered. As a result, the Lily device is a cap-like unit that incorporates Luminate’s new therapy and is worn by patients during and after each chemotherapy session, making it possible for chemotherapy-induced hair loss treatment to be delivered in a portable, comfortable and effective manner – allowing any patient, at any clinic, to receive hair loss prevention therapy.
Luminate Medical’s ambition extends beyond chemotherapy-induced hair loss. With their patented therapeutic approach, the team plans to develop further products to prevent other chemotherapy side-effects, such as neuropathy and infertility, and has even initiated research in NUI Galway to this effect. Luminate Medical’s extensive collaboration with NUI Galway also includes Prof Garry Duffy, Dr Alanna Stanley and Prof Aoife Lowery, complementing the team’s skillset with expert knowledge in Anatomy, Oncology and Clinical Research.
Following competitive and successful selection processes, the team participated in the most recent edition of Y Combinator, the US-based accelerator behind Airbnb, Dropbox and Stripe, and was the recipient of a €2.17M Disruptive Technologies Innovation Fund grant. Most recently, the team was also a winner at the 2021 Intertrade Ireland Seedcorn Investor Readiness and EIT Health Catapult competitions. All funding raised to date will allow the team to progress through device manufacture and clinical trials with patients.
For more details visit Luminate Medical
NUA Surgical
NUA Surgical is an award-winning start-up dedicated to innovating in women’s health. The company, founded in 2019 by Barry McCann, Padraig Maher and Marie-Therese Maher, is a spinout of BioInnovate Ireland and NUI Galway and is now deemed a HPSU (High Potential Start Up).

The unmet need tackled by NUA Surgical relates to birth by Caesarean Section (C-Section) surgery. Although this can be a life-saving procedure for both mother and infant, C-Section delivery is also associated with increased risk of complications for the mother such as infection, haemorrhage and even death when compared to a vaginal birth.
The venture started as a BioInnovate research project and received funding from Enterprise Ireland through the Commercialisation Fund. The team developed the SteriCISION C-Section Retractor, a device ergonomically designed for the obstetrician and their assistant, allowing hands-free unobstructed access to the uterus and delivering safer outcomes for mother and baby. Many people aren’t aware that C-Sections are the most common major surgery in the world accounting for 29 million births each year worldwide (Lancet, 2018). On average, a C-section is performed in 28% of births in Europe (source: Eurostat) and 32% of all births in the US (source: CDC) and the number of women being clinically indicated for c-section is continuously increasing.
“Through hundreds of clinician interviews, we have identified a true unmet clinical need. There is no gold standard retraction device recommended for C-Sections and obstetricians rely on old inadequate tools, or extra assistants,” said Barry McCann, CEO. To date, the company has completed three pre-clinical animal trials showing functionality and efficacy of the SteriCISION prototypes, it has conducted Human Factor testing at multiple hospitals and has carreid out extensive bench testing. The company also has a Quality Management System underway in accordance with US and EU regulations and is on track to reach design Select before the end of this year.
NUA Surgical’s founding team has almost 60 years combined industry experience covering commercial, technical and operations experience. McCann is an NUI Galway and BioInnovate Alum with almost 20 years of commercial and fundraising management experience, most recently within the MedTech industry. His transition to Medtech was accelerated by the BioInnovate Fellowship, a globally renowned needs-led innovation programme. He was also awarded with a JCI (Junior Chamber Ireland) Ten Outstanding Young Person’s Award for Medical Innovation in 2021.

Co-Founder and COO, Marie-Therese Maher is a Polymer Engineer and PMP certified Project Manager with 15+ years of experience in research and development at the medtech giant Medtronic. Co-Founder and CTO, Padraig Maher is a Polymer Engineer with 25+ years engineering and medical device manufacturing experience in medtech from J&J, Goodman, Abbott and Mednova.
This pre-revenue company has achieved multiple accolades, including Intertrade Ireland Seedcorn Winner 2020, EIT Health Headstart winner 2020, and Parkview Health ‘Global Maternal Health Innovation Competition’ winner 2021. At the end of 2021, NUA Surgical was also announced as a finalist in the prestigious Irish Medtech Awards. The founders were fortunate to secure additional funding through these awards and grants which has enabled the company to reach additional milestones before needing to raise equity investment.
The aim is to position its innovation as the new gold standard retractor for safe C-Sections which will require significant clinical data and is factored into its future roadmap. The next steps are to initially focus on the US market, with plans to establish its US office in the next 12 months, ahead of gaining FDA clearance.
For more information visit Nua Surgical.
Tympany Medical
Tympany Medical was founded in 2018 by BioInnovate Fellows Dr Liz McGloughlin and Rory O’Callaghan, has developed a next-generation endoscope platform for ENT surgery which solves unmet needs for patients, surgeons, and healthcare systems.

TheGalway-based MedTech Start-Up, has closed seed investment funding of over €3.5 million in a round involving several world-class MedTech investors. The seed investment will support the regulatory approval of the company’s first endoscope for ear surgery. Additionally, Tympany is advancing the development of its platform endoscope camera technology, which is at the cutting edge of mechanics, optics, electronics and human-centred design. The next generation technology will be designed to transition from the linear to the circular economy, placing Tympany Medical at the heart of sustainable innovation.
Ear surgery is performed in patients with conductive hearing loss. Over 660,000 ear surgeries are performed per year in the US and Europe. Since the founders completed the BioInnovate Fellowship in 2016, they have worked closely with key opinion leaders in ear surgery both in the Mayo Clinic in the US and Leiden University Medical Center in the Netherlands. The team is excited to continue pushing the boundaries of minimally invasive surgery and to advance the capabilities of endoscopy for ENT and beyond.
Tympany Medical has been supported to date by Enterprise Ireland via the Commercialisation Fund Programme, as well as EIT Health through its innovation project and Amplifier programmes and has been awarded support via the Disruptive Technology Innovation Fund.
For more information visit Tympany Medical
Venari Medical
Every year, 12 million US & EU citizens suffer from venous leg ulcers resulting from ineffective circulation known as Chronic Venous Disease (CVD) – a condition that doesn’t kill patients but does negatively impact their lives.

Established in 2018 by Stephen Cox, Dr Nigel Phelan and Sean Cummins, Venari Medical, a spin-out from BioInnovate, is focused on transforming the treatment of CVD sufferers – one of the most neglected and costly patient groups in healthcare.
CVD is the fourth most common chronic disease in the world, affecting one in every four adults and is increasingly common due to a rise in obesity and an aging population. This represents a large healthcare burden of up to 3% of Western healthcare budgets.
For sufferers, blood pools in their legs, which then leaks into surrounding tissue. Symptoms start with bulging dilated varicose veins, which progresses to pain and swelling, discolouration, breakdown of the skin, and malodorous leaking venous leg ulcers.
The goal of treatment is to close the diseased vein, allowing blood to circulate effectively in healthy veins, curing symptoms.
The market opportunity of CVD treatment is over $2 billion annually in the US & EU. Recent high-quality clinical evidence, supporting the benefits of acute intervention to improve overall healing of venous leg ulcers, has the potential to add over $1.5 billion to this opportunity. CVD management places a huge burden on healthcare systems amounting to $33 billion (€28 billion) per year in the US and EU alone, representing up to 3% of total healthcare expenditure.

In 2020, Venari closed a €4.5m international seed equity round to further development at their new offices at the Galway-Mayo Institute of Technology (GMIT) Innovation Hub, expand the team, and accelerate speed to market. The investment round was led by Nipro Corporation, a world-leading medical product manufacturer based in Osaka, Japan. The Western Development Commission and Enterprise Ireland also contributed to the investment, in addition to international medical device experts and vascular surgeons.
Venari’s EnVena device is currently in human pilot clinical trials with a further Series A investment funding round planned in 2022 ahead of planned regulatory submissions in the coming years required to reach the US, EU and Japanese markets.
Venari Medical has been further supported to this point by the Translational Medical Device Lab at NUI Galway led by Dr Martin O’Halloran. This relationship seeks to bridge the gap between industry and academia by adopting industry best-practice in device development, and supporting the medtech sector in both device design and clinical evaluation of novel devices.
Venari has been recognised internationally by a number of awards including EIT Health (2018 UK-Ireland Headstart funding winners), was a finalist in Medtech Innovator Accelerator Venari was also awarded ‘Best Early Stage’ company at the Connacht & Leinster Final of the 2019 InterTradeIreland Seedcorn Investor Readiness Competition which highlights high growth start-up companies on the island of Ireland.
For more information visit Venari Medical
ENDOWAVE
ENDOWAVE is a Galway-based, hi-tech medical device company that has its origins in the NUI Galway Bioinnovate programme. Its founders – Jonathan Boucher-Hayes (CEO), Jimmy Eaton-Evans (CTO) and Giuseppe Ruvio (CSO) spun-out from NUI Galway in 2020 to build a company to develop their pioneering microwave ablation technology for the treatment of lung cancer.
Lung cancer is the leading cause of all cancer deaths, with more than 2 million new patients diagnosed with lung cancer each year worldwide. With existing treatments regimes, the five-year survival rate for lung cancer patients remains dismal, at just 17%. Surgery is the current gold standard; however, up to 85% of patients are inoperable, often due to respiratory illness associated with smoking.
ENDOWAVE is developing a flexible microwave ablation catheter that can be delivered through the patient’s airways to directly destroy a targeted lung tumour. The minimally invasive procedure provides an alternative to surgery that is capable of delivering significant improvements to patient outcomes and recovery times, whilst reducing healthcare costs.
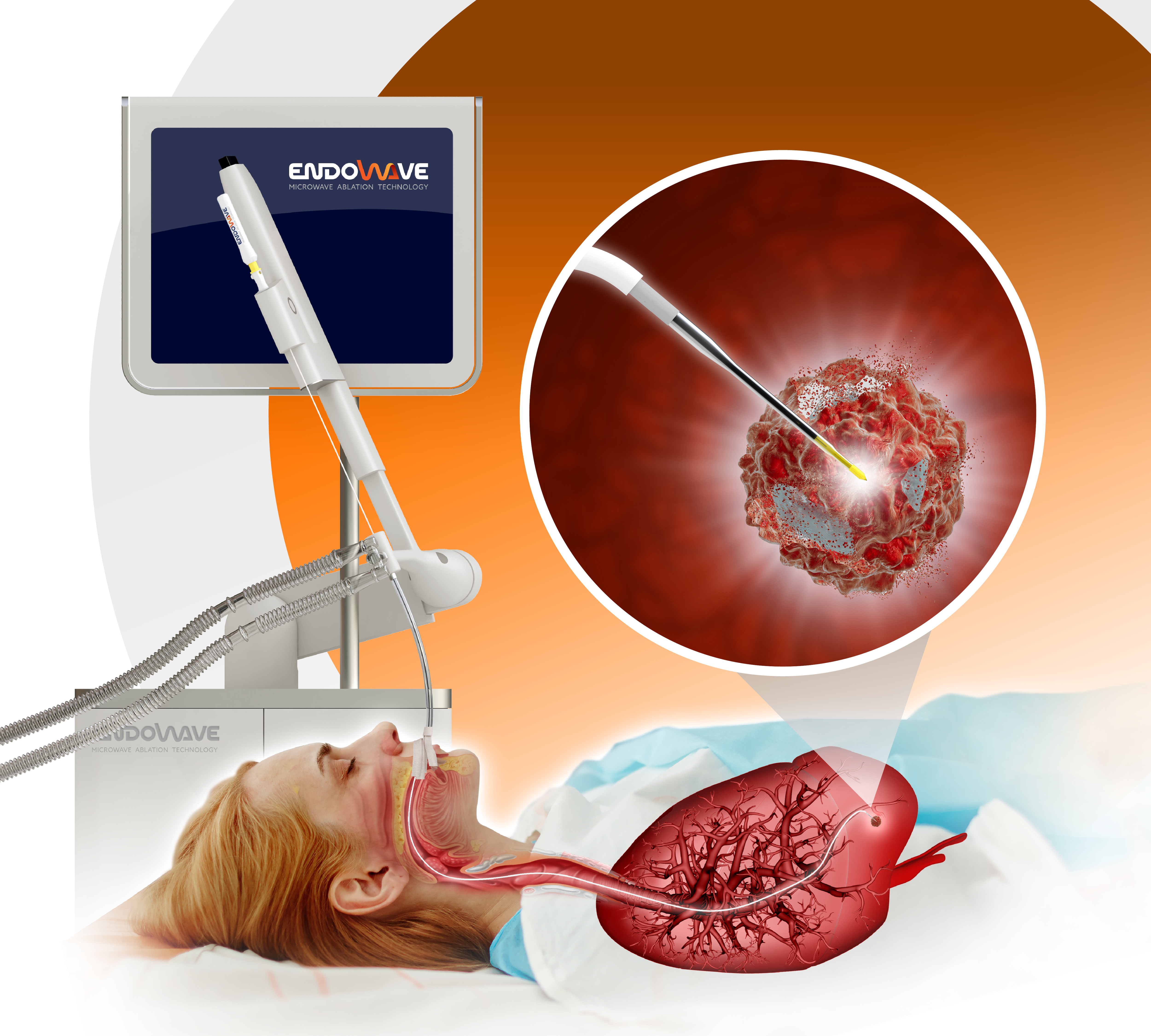
The ENDOWAVE FLEX catheter incorporates ground-breaking microwave antenna technology that enables the system to navigate the torturous path through the lung’s airways and deliver a controlled ablation zone that spares healthy tissue. The catheter technology is protected by four patent filings. Detailed numerical modelling and experimental testing has been carried out to optimise the device’s ablation and delivery performance.
ENDOWAVE have grown rapidly in the last 12 months, now employing 14 people with further expansion planned. The team has been awarded over €5.4m in grant funding to help commercialise their technology and build a pipeline of innovative products that will improve patient care and outcomes.
For more information visit Endowave
Profiles

NUI Galway Research Fellow, Bio Innovate, 2015

NUI Galway Researcher 2018-2020

NUI Galway Research Fellow, Bio Innovate, 2015